Scientific & Clinical Information
Auricular fibre Vagus Nerve Stimulation (afVNS) has been evaluated in scientific and clinical studies over several decades.
Vagus Nerve
The vagus nerve is the 10th cranial nerve (also known as ‘the great wondering protector’). It is a parasympathetic nerve with afferent and efferent sensory fibres and is the longest cranial nerve, with connections that allow control from high brain centres to key organs for maintaining the body’s balance (homeostasis). The vagus nerve has a wide range of reciprocal connections that allow for integration and responses to feedback signals in normal physiology.
Invasive Vagus Nerve Stimulation (iVNS)
VNS is a well-established clinical approach used for neuromodulation therapy for neurological conditions, such as drug-resistant epilepsy and depression.
Invasive VNS (iVNS) was originally approved in the USA for the treatment of epilepsy (1997) and then for medication-resistant depression (2005). VNS has been now studied and applied for a range of conditions including headache pain, arthritis, asthma, chronic pain, fibromyalgia, bipolar disorder and also aging-related diseases like dementia.
iVNS requires surgery that includes an incision in the patient’s neck conducted under general anaesthesia. An electrode is then inserted and attached around the vagus nerve. This is followed by insertion of an implanted pulse generator (IPG) under skin of the chest cavity of the patient.
iVNS is irreversible and has risks associated with surgery, such as infection (~3% of operations lead to device removal), nerve damage, voice alternations or vocal cord paralysis, as well as device malfunctioning such as wire breakages and disconnections.
afVNS Safety Profile
afVNS application holds a favourable risk-benefit ratio. The side effects experienced by users are typically ‘local’ i.e. at the site of electrode stimulation in the ear causing minor pain, redness or skin lesions. Other side effects can include 'systemic' effects where there are unwanted changes in the autonomic nervous system, which may occur in the form of headaches, nausea or vertigo.
The general safety and tolerability of afVNS has been previously reviewed [1]. With the inclusion of 51 scientific studies and a total of 1322 subjects, the occurrence of side effects have been well-characterised. afVNS is a safe and well-tolerated application.
[1] Redgrave et al Brain Stimul 2018;11(6):1225-1238
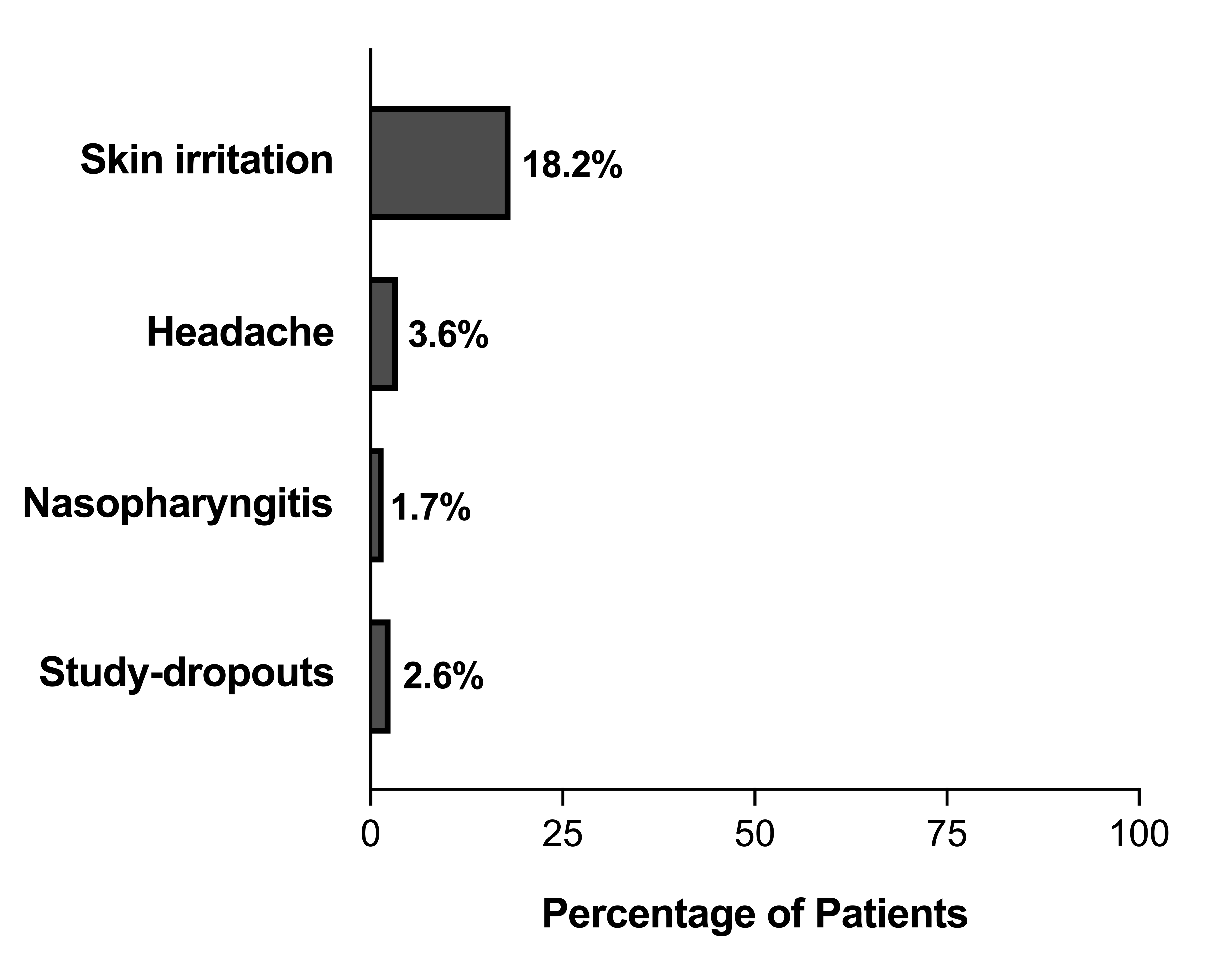
Migraine
Randomized Controlled Double Blind Study – The Journal of Headache and Pain (2015)
This study was a monocentric, prospective, double-blind, randomized, parallel group, controlled trial to assess the safety and efficacy of Auricular Vagus Nerve Stimulation as a preventative therapy for chronic migraines. The study was conducted in a tertiary outpatient clinic in Germany (department of neurology, University of Munich) between March 2012 and July 2014.
Chronic migraine patients between 18-70 years with a diagnosis according to the ICHD-IIR, ≥15 headache days per month, a duration of disease ≥6 months, no migraine-prophylactic medication or stable migraine-prophylactic medication for ≥1 month. A total of 46 patients were randomized to receive stimulation for 4 hours daily over 3 months.
The primary outcome measure in this study was mean change in headache days per 28 days. A headache day was defined as a calendar day with a headache of ≥4 h duration, or a headache successfully aborted by acute headache medication or any other treatment known to be typically effective for the patient. Secondary outcome parameters were: i. % of ‘responders’ (subjects having at least 50% reduction of headache days per 28 days from baseline), ii. change in mean headache intensity, iii. change in days with acute medication intake per 28 days, iv. change in headache–related disability as assessed by Migraine Disability Assessment (MIDAS) and Headache Impact Test (HIT-6) questionnaires, v. number and type of adverse events.
Of 46 randomized patients, 40 completed the study (per protocol). Patients that received 1 Hz stimulation had significantly larger reduction in the headache days per 28 days than patients receiving 25 Hz stimulation (-7.0 ± 4.6, 36% reduction from baseline vs. -3.3 ± 5.4 days, 17% reduction from baseline; p=0.035, respectively). It was also found that 29.4% of patients receiving 1 Hz stimulation had a ≥50% reduction in headache days vs. 13.3% in the 25 Hz stimulation group. The number of days with intake of acute headache medication were significantly reduced in both groups. Headache intensity was not significantly changed in either treatment group and there were no differences.
HIT-6 and MIDAS scores were significantly improved in both groups. Among the patients treated with stimulation, there was high compliance, with the average number of stimulated hours per day being around 3.4 hours in all groups, corresponding to ~85% of the requested stimulation time.
No serious treatment related adverse events were found. Most adverse events were mild or moderate in severity and resolved without sequelae. The most frequent adverse events were mild or moderate pain at the site of stimulation, paresthesia or pruritus during or after stimulation, erythema, ulcer or scabbing.
In conclusion, treatment of chronic migraine by 1 Hz stimulation was safe and effective with a higher superiority than 25 Hz stimulation for reducing chronic migraines. Auricular Vagus Nerve Stimulation treatment improves quality of life in chronic migraine patients and offers a patient compliant therapeutic option with good tolerability and safety. The efficacy of stimulation shown in this study is comparable to gold-standard medicative treatments used for migraine.

Auricular Vagus Nerve Stimulation for Better Aging
Research on Auricular Vagus Nerve Stimulation in Aging – Aging (2019)
This research examined the impact of auricular vagus nerve stimulation on autonomic function and well-being in individuals aged 55 years and older. The study included three separate investigations, focusing on both single-session and daily use of auricular vagus nerve stimulation over two weeks, assessing heart rate variability (HRV), baroreflex sensitivity (BRS), quality of life (QoL), mood, and sleep quality.
Aging is typically associated with a shift in autonomic balance, characterized by increased sympathetic and reduced parasympathetic activity. These changes can negatively impact cardiovascular health, emotional well-being, and cognitive function, and may contribute to age-related conditions such as hypertension, heart failure, and depression. Enhancing parasympathetic activity using auricular vagus nerve stimulation has emerged as a promising non-invasive approach to mitigating these age-related issues.
Participants underwent either a single session or daily 15-minute auricular vagus nerve stimulation for two weeks. HRV and BRS were measured to evaluate autonomic function, while subjective assessments of QoL, mood, and sleep were collected.
The results were as follows:
-
Autonomic Improvements: Both single-session and two-week daily auricular vagus nerve stimulation use increased measures of vagal tone (e.g., HRV) and improved autonomic balance. Participants with higher baseline sympathetic activity showed more significant shifts toward parasympathetic dominance.
-
Enhanced Baroreflex Sensitivity: auricular vagus nerve stimulation significantly improved BRS, a marker linked to reduced cardiovascular mortality risk, suggesting potential cardiovascular benefits.
-
Mood and Quality of Life: Two weeks of daily auricular vagus nerve stimulation improved mood parameters (e.g., reduced depression and tension) and aspects of QoL. Participants with higher baseline levels of stress or depression experienced the most pronounced improvements.
-
Better Sleep Quality: Participants reported enhancements in sleep quality, ease of falling asleep, and ease of waking up, particularly those who initially struggled with these aspects.
The study indicates that auricular vagus nerve stimulation could play a crucial role in promoting healthier aging by addressing autonomic imbalances, improving emotional well-being, and enhancing sleep quality. This non-invasive therapy may prolong the period of healthy aging, reduce medication dependency, and improve overall quality of life in older adults.
Auricular vagus nerve stimulation is a promising intervention for mitigating age-related declines in autonomic function and enhancing well-being. Future research should explore long-term effects and optimal stimulation parameters for various age-related conditions, potentially offering a safe and effective tool for promoting healthy aging.
Auricular Vagus Nerve Stimulation for Alleviating Anxiety
Double Blind, Randomized, Controlled Clinical Trial – Frontiers in Integrative Neuroscience (2024)
This double-blind, randomized, and controlled trial aimed to evaluate the efficacy and safety of auricular vagus nerve stimulation for the reduction of anxiety symptoms. Conducted at a leading research institution in Portugal, the study focused on assessing how non-invasive vagus nerve stimulation could be a viable therapy for individuals suffering from anxiety.
The study recruited 68 adults aged 18-60 years who were diagnosed with Generalized Anxiety Disorder (GAD) based on DSM-5 criteria. Participants were randomly assigned to receive either active stimulation or a sham (placebo) treatment. The intervention involved daily 30-minute sessions over four weeks, targeting the auricular branch of the vagus nerve. Anxiety levels were measured using validated scales, including the Beck Anxiety Inventory (BAI) and State-Trait Anxiety Inventory (STAI), both before and after the intervention period, with follow-up assessments conducted four weeks post-treatment.
The key outcomes and findings of the study were as follows:
Significant Anxiety Reduction: Participants in the active stimulation group demonstrated a statistically significant decrease in BAI scores compared to the sham group, indicating that auricular vagus nerve stimulation effectively reduced anxiety symptoms. The STAI scores also reflected marked improvements, highlighting the therapy’s positive impact on both state and trait anxiety.
-
Sustained Benefits: The anxiety reduction effects observed in the active stimulation group were maintained at the four-week follow-up, suggesting that auricular vagus nerve stimulation provides enduring benefits beyond the treatment period.
-
Physiological Improvements: Active stimulation was associated with enhanced heart rate variability (HRV), a marker of autonomic nervous system balance. Increased HRV indicates better vagal tone and reduced sympathetic nervous system dominance, which are crucial for anxiety management and overall stress resilience.
-
Safety and Tolerability: The stimulation treatment was well-tolerated, with no serious adverse effects reported. Mild and temporary side effects, such as slight ear discomfort, were noted in a small number of participants but resolved quickly.
This study shows how auricular vagus nerve stimulation can be used as a non-invasive, drug-free intervention for managing anxiety. By modulating the autonomic nervous system and improving vagal tone, auricular vagus nerve stimulation offers a promising therapeutic option that may benefit individuals seeking alternatives to traditional pharmacological treatments. The sustained reduction in anxiety symptoms and the favorable safety profile of neurostimulation underscore its viability for long-term use.
Auricular Vagus Nerve Stimulation for Improving Sleep
Sleep Improvement Study Using Auricular Vagus Nerve Stimulation – Autonomic Neuroscience: Basic and Clinical (2022)
This randomized, single-blind, controlled clinical trial examined the impact of a two-week course of daily stimulation on global sleep quality in adults. The trial included 78 community-dwelling participants aged 18 to 75 years. Participants were divided into two main groups (early and late auricular vagus nerve stimulation ) and further randomized into active stimulation and sham (control) conditions.
Participants received stimulation for 4 hours daily for 14 days, and the effects on sleep were measured using the Pittsburgh Sleep Quality Index (PSQI). The primary outcome was the change in global sleep scores over time.
The key findings of the study were as follows:
-
Participants receiving active stimulation showed significant improvements in global sleep scores during both the early and late intervention phases (C = -1.90 and C = -0.87, respectively).
-
The study suggests potential biological mechanisms, including increased parasympathetic activity and reduced inflammation and cortisol levels, that may underlie the sleep benefits observed with auricular vagus nerve stimulation.
Improving Cognition in Aging with Auricular Vagus Nerve Stimulation
Randomized Controlled Double-Blind Study – Brain Stimulation (2022)
This double-blind, randomized controlled trial assessed the safety and efficacy of auricular vagus nerve stimulation in improving cognitive function in patients with mild cognitive impairment (MCI).
Participants aged 55–75 years diagnosed with MCI were randomized into two groups: (a) taVNS group received stimulation targeting the auricular vagus nerve distribution (b) sham taVNS group received stimulation at non-vagus nerve region.
Participants received stimulation 30-minute sessions twice daily, five days a week, for 24 weeks.
Cognitive Function: The auricular vagus nerve stimulation group showed significant improvements in cognitive performance of Montreal Cognitive Assessment-Basic (MoCA-B) scores compared to the sham group (mean increase of 3.24 points vs. 0.33 points).
Memory functions: specifically immediate recall (N5) and delayed recall (N7), improved significantly in this group.
Secondary Outcomes: Language and executive functions were positively impacted in the auricular vagus nerve stimulation group.
Sleep quality also showed significant improvement in the auricular vagus nerve stimulation group, suggesting a beneficial effect on restorative sleep, which is critical for cognitive health.
Conclusion
Auricular vagus nerve neurostimulation enhances memory recall, language abilities, and overall cognitive performance while also improving sleep quality.
Reducing Symptoms of Inflammatory Bowel Disease
Proof-of-Concept Study – Bioelectronic Medicine (2023)
This proof-of-concept study effects of auricular vagus nerve stimulation for improving mild to moderate inflammatory bowel diseases (IBD) including Crohn’s disease (CD) and ulcerative colitis (UC) in young adults and paediatric patients.
Participants underwent auricular vagus nerve stimulation in a two-phase design: a sham-controlled crossover phase (4 weeks) followed by an active stimulation treatment phase (14 weeks). The primary outcomes included reductions in fecal calprotectin (FC), a key biomarker of intestinal inflammation, and clinical remission rates.
Reduction in Inflammation: 64.7% of participants achieved a ≥50% reduction in FC by Week 16. Median reductions in FC were 51% for CD and 81% for UC, with several participants reaching normalized FC levels.
Clinical Symptom Improvements: 50% of CD patients and 33% of UC patients achieved clinical remission.
Emotional Well-being: Significant reductions in anxiety scores were reported on patient reported outcomes (PROMIS) questionnaires.
Vagal Activity: HRV analysis revealed increased vagal tone in participants with low baseline levels, demonstrating effective autonomic modulation
Conclusion
This study highlights auricular vagus nerve stimulation as a promising non-invasive therapy for pediatric IBD, providing reductions in intestinal inflammation, improved clinical outcomes, and enhanced emotional well-being. By activating the brain-gut axis, auricular vagus nerve stimulation represents a safe and innovative approach to managing chronic inflammatory bowel conditions.
Clinical Efficacy in the Treatment of Chronic Pain
Systematic Review and Meta-Analysis – Pain Therapy Journal (2024)
This systematic review and meta-analysis examined the clinical efficacy and safety of auricular vagus nerve stimulation in addressing chronic pain conditions. The studies analyzed included various chronic pain indications such as back pain, migraine, fibromyalgia, and abdominal pain.
Summary of Key Findings:
Primary Outcomes: A significant reduction in visual analog scale (VAS) pain intensity was observed in chronic pain patients treated with aVNS compared to sham or control treatments.
•Percutaneous VNS (pVNS): Demonstrated greater efficacy, with effect sizes indicating more significant pain reductions due to the closer proximity of needle electrodes to the nerve.
•Auricular vagus nerve stimulation: Also effective, showing improvements across chronic conditions but with slightly lower effect sizes.
Secondary Outcomes: Improvements in quality-of-life metrics, including reductions in fatigue, better sleep, and enhanced psychological well-being, were reported.
Chronic Conditions Analyzed:
•Migraine: Reduction in headache frequency and intensity.
•Back Pain: Significant alleviation of pain symptoms.
•Abdominal Pain: Marked improvement in functional and symptomatic measures.
Therapeutic Mechanisms: Evidence suggests that auricular vagus nerve stimulation modulates brain activity in pain-processing centers, such as the prefrontal cortex and thalamus, while activating parasympathetic responses and anti-inflammatory pathways.
Safety: auricular vagus nerve stimulation was well-tolerated with mild and transient side effects, including skin irritation and fatigue.
Implications for Chronic Pain Management:
Auricular vagus nerve stimulation presents a safe and effective non-drug treatment option for chronic pain conditions. It provides a complementary approach to traditional therapies, enabling sustainable reductions in pain intensity and improvements in overall quality of life. With further standardization in treatment protocols, auricular vagus nerve stimulation holds potential for broader adoption in pain management strategies worldwide.
Reducing Long-Covid Symptoms
Pilot Randomized Controlled Trial – Bioelectronic Medicine (2022)
This study explored the safety, feasibility, and potential symptom relief of a self-administered auricular vagus nerve stimulation for managing long-COVID symptoms. The trial included 12 participants who self-administered the therapy twice daily for four weeks, focusing on addressing nine symptoms: anxiety, depression, fatigue, irritability, brain fog, vertigo, anosmia, ageusia, and headaches.
Key Results
The study found that auricular vagus nerve stimulation is safe and feasible for at-home use, with no adverse effects reported. Participants showed a trend toward reduced long-COVID symptoms, with notable improvements in mental fatigue among those receiving the active treatment for the full four-week period.
Conclusion
This trial demonstrates that auricular vagus nerve stimulation is a promising intervention for mitigating the prolonged neuropsychiatric and physical symptoms of long COVID and is a safe, effective, and accessible therapeutic tool for individuals managing the long-term impacts of COVID-19.