The Science of
Vagus Nerve Stimulation (VNS)
Vagus Nerve stimulation can be achieved non-invasively through targeting the auricular fibers in the ear. The Vagus Nerve is a key cranial nerve in the nervous system that carries signals on the body’s state of balance (homeostasis) to- and from- the brain.
In common health conditions, there is often an imbalance of these signals causing underlying discomfort and disease symptoms.
With auricular fiber Vagus Nerve Stimulation (afVNS) therapy, carefully bioengineered electrical pulses are used to modulate the Vagus Nerve to naturally rebalance the nervous system for improving health.
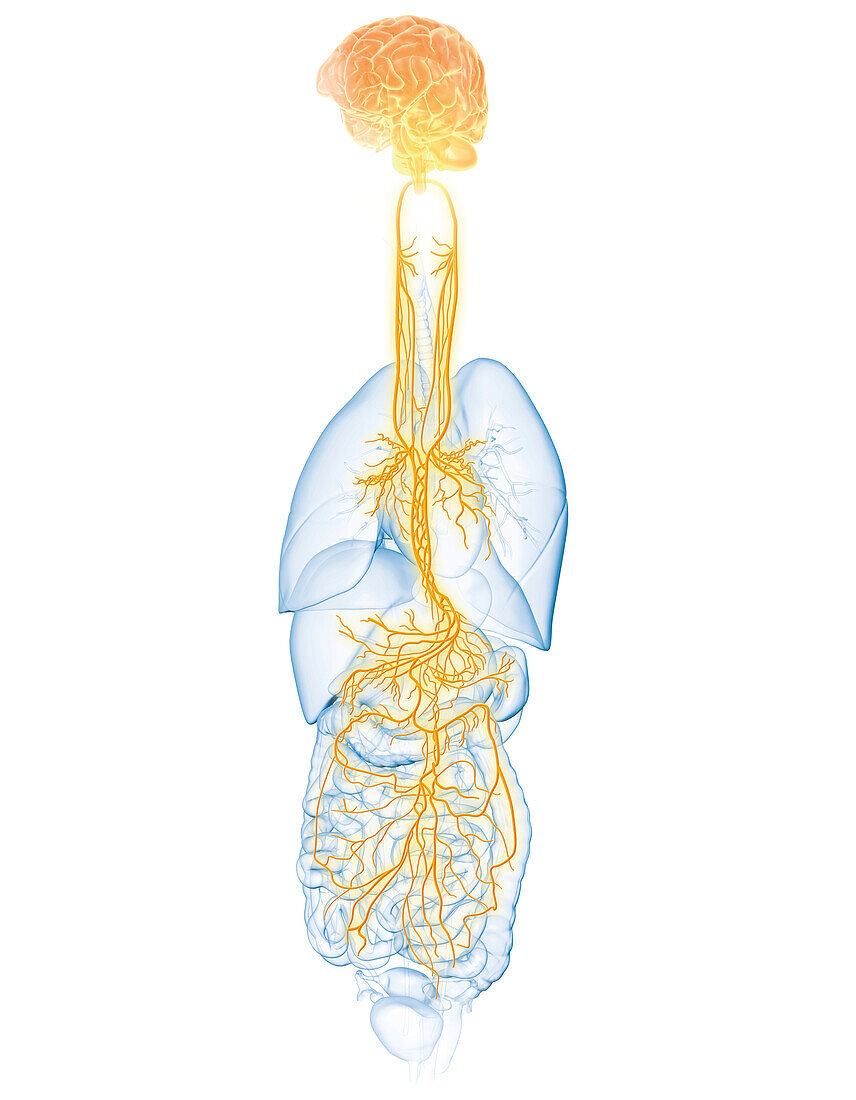

Neural Mechanisms of afVNS
afVNS activates sensory (A-Beta) nerve fibers in the ear stimulating the auriculo-vagal neural pathways. This guides the nervous system towards a healthier state from a parasympathetic (aka ’rest & digest’) response.
Users of afVNS therapy choose a drug-free non-invasive application to improve their health disorder, without experiencing troublesome side effects that is often caused by drug chemicals.
Neuropix afVNS Technology
Neuropix’s technology delivers an optimized bioelectrical formulae to activate auricular fibers of the vagus nerve, achieving better health outcomes in a shorter period of time.
Our bioengineering has been carefully developed to maximize performance, making it an advanced, user-centric solution for improved health and well-being.

Scientific Data
Neuropix neurostimulation is based on over a decade of scientific and clinical research showing auricular fibre Vagus Nerve Stimulation (afVNS) is a safe & feasible application. Research publications have demonstrated the therapeutic benefits for improved health showing that afVNS is an alternative to medication that is safe and accessible.
Clinical results may vary

Health Indications
afVNS therapy is used for reducing anxiety & stress, sleep disturbances and healthier aging. Please note that afVNS is not an acute therapy and may require several weeks to take effect. It is a self-applied therapy and can be used at the convenience of the user. For full healthcare indications, please contact the Neuropix team for further information.
Safety Information
afVNS therapy should not be used in pregnant women, persons with active implants (e.g. cochlear implants, vagus nerve stimulators or pacemakers), cerebral shunts or applied to disease skin. Users with underlying health conditions affecting the cardiac system should ask their medical doctor whether afVNS is suitable for them. For full use instructions and safety information, please see user manuals.