科學與臨床資訊
耳廓纖維迷走神經刺激(afVNS)已在科學和臨床研究中評估了十多年。
迷走神經
迷走神經是第10對顱神經(也被稱為“偉大的遊走保護者”)。它是一種副交感神經,具有傳入和傳出感覺纖維,是最長的顱神經。迷走神經通過連接高腦中樞與關鍵器官,幫助維持身體的平衡(內穩態)。迷走神經具有廣泛的雙向連接,能夠在正常生理中實現反饋信號的整合與響應。
侵入性迷走神經刺激(iVNS)
迷走神經刺激(VNS)是一種成熟的臨床方法,用於治療神經系統疾病的神經調節療法,例如難治性癲癇和抑鬱症。
侵入性迷走神經刺激(iVNS)最初於1997年在美國被批准用於治療癲癇,隨後於2005年被批准用於治療藥物難治性抑鬱症。目前,VNS已被研究並應用於多種疾病,包括頭痛、關節炎、哮喘、疼痛、纖維肌痛、雙相情感障礙以及與衰老相關的疾病如癡呆症。
iVNS需要進行手術,包括在患者頸部進行切口並在全身麻醉下操作。隨後,將電極插入並固定在迷走神經周圍。接著,將植入式脈衝發生器(IPG)植入患者胸腔皮膚下。
iVNS是不可逆的,並伴有手術相關的風險,例如感染(約3%的手術會導致設備移除)、神經損傷和聲音變化/聲帶麻痺,以及設備故障,例如電線斷裂和連接問題。
afVNS 安全性概況
afVNS 應用具有良好的風險-收益比。用戶通常經歷的副作用是“局部的”,即電極在耳部刺激部位可能引起輕微疼痛、發紅或皮膚損傷。其他副作用可能包括“系統性”影響,例如自主神經系統的變化,這可能表現為頭痛、噁心或暈眩。
afVNS 的整體安全性和耐受性曾被研究評估[1]。通過納入51項科學研究和總計1322名受試者,副作用的發生已被充分表徵。afVNS 是一種安全且耐受性良好的應用。
[1] Redgrave et al Brain Stimul 2018;11(6):1225-1238
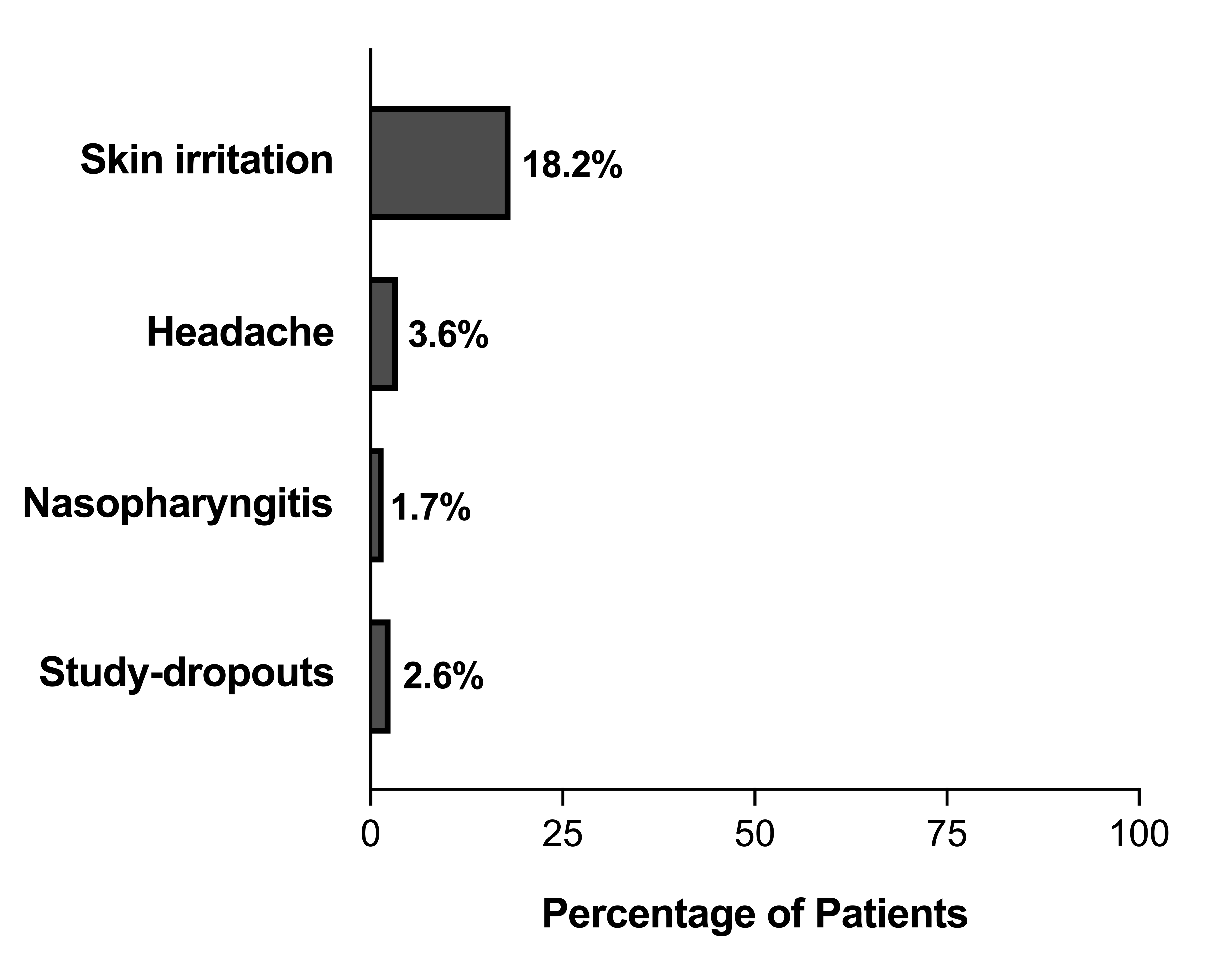
偏頭痛
隨機對照雙盲研究 – 《頭痛與疼痛雜誌》(2015)
本研究是一項單中心、前瞻性、雙盲、隨機、平行組對照試驗,旨在評估耳廓迷走神經刺激作為慢性偏頭痛預防療法的安全性和有效性。研究於2012年3月至2014年7月在德國的一家三級門診(慕尼黑大學神經病學系)進行。
研究對象為年齡在18至70歲之間、根據ICHD-IIR診斷為慢性偏頭痛的患者(每月頭痛天數≥15天、病程≥6個月),且未接受偏頭痛預防藥物治療或已使用穩定的偏頭痛預防藥物≥1個月。共46名患者被隨機分組,每天接受4小時的刺激治療,為期3個月。
本研究的主要結果指標為每28天頭痛天數的平均變化。頭痛天數定義為持續≥4小時的頭痛日,或通過急性頭痛藥物或其他患者已知有效治療成功終止的頭痛日。次要結果參數包括:
- “應答者”的百分比(從基線起頭痛天數減少≥50%的患者);
- 平均頭痛強度的變化;
- 每28天使用急性藥物的天數變化;
- 根據偏頭痛殘疾評估(MIDAS)和頭痛影響測試(HIT-6)問卷評估的與頭痛相關的殘疾變化;
- 不良事件的數量和類型。
在46名隨機分組患者中,40名完成了研究(按方案分析)。接受1 Hz刺激的患者每28天的頭痛天數顯著減少,與接受25 Hz刺激的患者相比有更大降幅(-7.0 ± 4.6天,基線減少36% vs. -3.3 ± 5.4天,基線減少17%;p=0.035)。此外,接受1 Hz刺激的患者中有29.4%頭痛天數減少≥50%,而25 Hz刺激組這一比例為13.3%。兩組患者的急性頭痛藥物使用天數均顯著減少。頭痛強度在兩組治療中均無顯著變化。
HIT-6和MIDAS評分在兩組中均顯著改善。接受刺激治療的患者依從性較高,所有組每日平均刺激時間約為3.4小時,相當於要求刺激時間的~85%。
未發現嚴重與治療相關的不良事件。大多數不良事件為輕度或中度,且無後遺症。最常見的不良事件為刺激部位的輕度或中度疼痛、刺痛感或癢感,以及刺激期間或之後的紅斑、潰瘍或結痂。
總之,1 Hz刺激治療慢性偏頭痛是安全且有效的,其療效優於25 Hz刺激。耳廓迷走神經刺激治療能夠改善慢性偏頭痛患者的生活質量,提供了一種患者依從性高且耐受性和安全性良好的治療選擇。本研究顯示的刺激療效可與用於偏頭痛的金標準藥物治療相媲美。

耳廓迷走神經刺激改善衰老
耳廓迷走神經刺激對衰老的研究 – Aging (2019)
本研究探討了耳廓迷走神經刺激對55歲及以上人群自主神經功能和健康狀態的影響。研究分為三個獨立的實驗,分別研究了單次使用和每天使用耳廓迷走神經刺激兩週的效果,評估了心率變異性(HRV)、壓力感受器反射敏感性(BRS)、生活質量(QoL)、情緒和睡眠質量。
衰老通常與自主神經平衡的變化相關,表現為交感神經活動增加和副交感神經活動減少。這些變化可能對心血管健康、情緒和認知功能產生負面影響,並可能導致與年齡相關的疾病,如高血壓、心力衰竭和抑鬱症。通過耳廓迷走神經刺激增強副交感神經活動,已成為一種有前景的非侵入性方法,能夠緩解這些與年齡相關的問題。
參與者接受了單次15分鐘刺激或連續兩週每天15分鐘的耳廓迷走神經刺激。研究測量了HRV和BRS來評估自主神經功能,同時收集了生活質量、情緒和睡眠的主觀評估數據。
研究結果如下:
- 自主神經改善:單次使用和連續兩週每天使用耳廓迷走神經刺激均提高了迷走神經張力的指標(如HRV)並改善了自主神經平衡。交感神經活動較高的參與者表現出向副交感神經占優的顯著轉變。
- 增強壓力感受器反射敏感性:耳廓迷走神經刺激顯著改善了BRS,這一指標與降低心血管死亡風險相關,表明其可能具有心血管健康益處。
- 改善情緒和生活質量:連續兩週每天使用耳廓迷走神經刺激改善了情緒指標(如減少抑鬱和緊張)以及生活質量的某些方面。高壓力或抑鬱水平的參與者改善最為明顯。
- 提高睡眠質量:參與者報告睡眠質量、入睡難度和醒來容易度均有所改善,尤其是那些起初在這些方面有困難的參與者。
研究表明,耳廓迷走神經刺激可通過改善自主神經失衡、提升情緒健康以及提高睡眠質量,在促進健康衰老方面發揮重要作用。這種非侵入性療法可能延長健康老齡化的時間,減少藥物依賴,並提高老年人的整體生活質量。
耳廓迷走神經刺激是一種有前景的干預措施,可以緩解與年齡相關的自主神經功能下降並增強健康狀態。未來研究應探索其長期效果和針對不同年齡相關疾病的最佳刺激參數,從而為促進健康衰老提供安全有效的工具。
耳廓迷走神經刺激緩解焦慮
雙盲、隨機、對照臨床試驗 – 《整合神經科學前沿》(2024)
本雙盲、隨機、對照試驗旨在評估耳廓迷走神經刺激在減少焦慮症狀方面的療效和安全性。該研究在葡萄牙一家領先的研究機構進行,重點評估非侵入性迷走神經刺激作為焦慮症患者可行治療方案的潛力。
研究招募了68名年齡在18至60歲之間的成年人,這些參與者均根據DSM-5標準被診斷為廣泛性焦慮障礙(GAD)。參與者被隨機分配到接受主動刺激或假刺激(安慰劑)治療的組別。干預涉及為期四週的每日30分鐘刺激,目標是迷走神經耳廓分支。在干預前後以及治療結束四週後,使用經過驗證的量表(包括貝克焦慮量表BAI和狀態-特質焦慮量表STAI)測量焦慮水平。
研究的主要結果和發現如下:
- 顯著減少焦慮:主動刺激組的參與者在BAI評分方面較假刺激組顯示出統計學上顯著的下降,表明耳廓迷走神經刺激有效減少了焦慮症狀。STAI評分也顯示顯著改善,凸顯了該療法對狀態焦慮和特質焦慮的積極影響。
- 持久的效果:主動刺激組觀察到的焦慮緩解效果在治療結束四週後的隨訪中得以維持,表明耳廓迷走神經刺激的益處可持續。
- 生理改善:主動刺激與心率變異性(HRV)的增強相關,這是自主神經系統平衡的標誌。HRV的增加表明迷走神經張力改善、交感神經系統主導性降低,這對焦慮管理和壓力恢復力至關重要。
- 安全性和耐受性:刺激治療耐受性良好,未報告嚴重不良反應。少數參與者出現了輕微的耳部不適等短暫副作用,但均迅速恢復。
該研究表明,耳廓迷走神經刺激可作為管理焦慮的非侵入性、無藥物干預手段。通過調節自主神經系統並改善迷走神經張力,耳廓迷走神經刺激為尋求傳統藥物治療替代方案的個體提供了一種有前景的治療選擇。焦慮症狀的持續改善以及神經刺激的良好安全性進一步證明了其長期使用的可行性。
耳廓迷走神經刺激改善睡眠
使用耳廓迷走神經刺激的睡眠改善研究 – 《自主神經科學:基礎與臨床》(2022)
這項隨機、單盲、對照臨床試驗探討了為期兩週的每日刺激療程對成年人整體睡眠質量的影響。試驗共招募了78名社區居住者,年齡在18至75歲之間。參與者分為兩組(早期和晚期耳廓迷走神經刺激),並進一步隨機分配至主動刺激組和假刺激(對照)組。
參與者每天接受4小時的刺激,為期14天,並通過匹茲堡睡眠質量指數(PSQI)評估睡眠效果。主要研究結果為整體睡眠評分隨時間的變化。
研究的主要發現如下:
- 接受主動刺激的參與者在早期和晚期干預階段的整體睡眠評分均顯著改善(C = -1.90 和 C = -0.87,分別對應)。
- 研究表明,可能的生物學機制包括副交感神經活動增強、炎症和皮質醇水平降低,這些機制可能是耳廓迷走神經刺激對睡眠改善效果的基礎。
通過耳廓迷走神經刺激改善老年人的認知障礙
隨機對照雙盲研究 – 《腦刺激》(2022)
這項雙盲隨機對照試驗評估了耳廓迷走神經刺激在改善輕度認知障礙(MCI)患者認知功能方面的安全性和有效性。
55-75歲被診斷為MCI的參與者被隨機分為兩組:
(a) taVNS組 接受針對耳廓迷走神經分佈的刺激
(b) 假taVNS組 接受非迷走神經區域的刺激
參與者每天接受兩次30分鐘的刺激,每週五天,持續24週。
- 認知功能:耳廓迷走神經刺激組在蒙特利爾認知評估-基礎版(MoCA-B)評分上的認知表現顯著提高,相比假刺激組,平均提升3.24分,而假組僅提升0.33分。
- 記憶功能:特別是在即時回憶(N5)和延遲回憶(N7)方面,該組有顯著改善。
- 次要結果:耳廓迷走神經刺激組在語言和執行功能方面也有積極影響。
- 睡眠質量:耳廓迷走神經刺激組的睡眠質量顯著改善,表明對恢復性睡眠有益,而恢復性睡眠對認知健康至關重要。
結論:耳廓迷走神經刺激能夠增強記憶回憶、語言能力和整體認知表現,同時也改善了睡眠質量。
減少炎症性腸病症狀
概念驗證研究 – 生物電子醫學(2023)
這項概念驗證研究評估了耳廓迷走神經刺激對改善輕中度炎症性腸病(IBD),包括克羅恩病(CD)和潰瘍性結腸炎(UC),在年輕人和兒科患者中的效果。
參與者接受了兩階段的耳廓迷走神經刺激:一個為期4周的假刺激對照交叉階段,接著是為期14周的主動刺激治療階段。主要結果包括糞便鈣衛蛋白(FC)水平的下降——這是腸道炎症的關鍵生物標誌物——以及臨床緩解率。
- 炎症減少:64.7%的參與者在第16周達到了FC水平降低≥50%。CD患者的FC中位下降為51%,UC患者為81%,其中一些參與者的FC水平恢復正常。
- 臨床症狀改善:50%的CD患者和33%的UC患者達到了臨床緩解。
- 情緒健康:患者報告結果(PROMIS問卷)顯示焦慮評分顯著下降。
- 迷走神經活動:心率變異性(HRV)分析顯示,低基礎水平的參與者迷走神經張力增加,表明自主神經調節效果顯著。
結論:研究表明,耳廓迷走神經刺激是一種有前景的非侵入性療法,用於兒科IBD的管理,能夠減少腸道炎症,改善臨床結果,並增強情緒健康。通過激活腦腸軸,耳廓迷走神經刺激為慢性炎症性腸病的管理提供了一種安全且創新的解決方案。
耳廓迷走神經刺激在治療慢性疼痛中的臨床療效
系統評價與匯總分析 – 《疼痛治療雜誌》(2024)
這項系統評價與匯總分析研究了耳廓迷走神經刺激(aVNS)在應對慢性疼痛狀況中的臨床療效和安全性。分析的研究涵蓋了多種慢性疼痛類型,包括背痛、偏頭痛、纖維肌痛和腹痛。
主要研究結果總結
- 主要結果:與假刺激或對照治療相比,接受aVNS治療的慢性疼痛患者的視覺模擬量表(VAS)疼痛強度顯著降低。
- 經皮迷走神經刺激(pVNS):效果更顯著,針電極更接近神經的部位帶來了更大的疼痛緩解效果。
- 耳廓迷走神經刺激:在慢性疾病中同樣有效,顯示了整體改善,但效果略低於pVNS。
次要結果:生活質量指標得到改善,包括疲勞減輕、更好的睡眠和心理健康的提升。
分析的慢性病狀況
- 偏頭痛:頭痛頻率和強度顯著降低。
- 背痛:疼痛症狀顯著緩解。
- 腹痛:功能和症狀指標顯著改善。
治療機制:證據表明,耳廓迷走神經刺激通過調節大腦疼痛處理中心(如前額葉皮質和丘腦)的活動,同時激活副交感神經反應和抗炎途徑來發揮作用。
安全性:耳廓迷走神經刺激耐受性良好,僅有輕微且短暫的副作用,包括皮膚刺激和疲勞。
慢性疼痛管理的意義
耳廓迷走神經刺激為慢性疼痛提供了一種安全有效的非藥物治療選擇。它是傳統療法的補充手段,能夠實現疼痛強度的持續性降低並改善整體生活質量。隨著治療方案的進一步標準化,耳廓迷走神經刺激有望在全球範圍內的疼痛管理策略中得到更廣泛的應用。
使用耳廓迷走神經刺激療法緩解長期新冠症狀
試點隨機對照試驗 – 生物電子醫學 (2022)
本研究探討了一種可自行操作的耳廓迷走神經刺激療法在管理長期新冠症狀方面的安全性、可行性及潛在的症狀緩解效果。試驗包括12名參與者,他們每天自行操作該療法兩次,持續四週,重點針對九種症狀:焦慮、抑鬱、疲勞、易怒、腦霧、眩暈、嗅覺喪失、味覺喪失以及頭痛。
關鍵結果: 研究發現耳廓迷走神經刺激在家中使用是安全且可行的,未報告任何不良反應。參與者的長期新冠症狀有減輕趨勢,尤其是接受完整四週活躍治療的患者,其精神疲勞顯著改善。
結論: 本次試驗表明,耳廓迷走神經刺激是一種有前景的干預方法,可以減輕長期新冠的神經精神及身體症狀,是一種安全、有效且可及的療法,用於幫助個體管理新冠的長期影響。